
Frequently Asked Questions (FAQs)
- It is sealed using special pressure valves and should never be opened
- Typically uses Calcium/Calcium metal alloys
- It is maintenance-free with proper charge controls
- Uses a recombination reaction to prevent the escape of hydrogen and oxygen gases normally lost in a flooded lead-acid battery (particularly in deep cycle applications)
- It is sealed using special pressure valves and should never be opened
- Typically uses Calcium/Calcium metal alloys.
- With proper charge controls is maintenance-free
- Has its entire electrolyte absorbed in separators consisting of a sponge-like mass of matted glass fibers
- Uses a recombination reaction to prevent the escape of hydrogen and oxygen gases normally lost in a flooded lead-acid battery
A Flooded battery is a type of lead acid battery in which the electrolyte is in movable liquid state
- Can be both sealed and open-vented
- Can use High Antimony, Low Antimony or Calcium metal alloys or a combination of Calcium and Low Antimony grids (hybrid)
- Requires maintenance in cyclic applications
- Its entire electrolyte volume is free to move within the cell with nothing to prevent the escape of hydrogen and oxygen gases normally lost during charging and discharging (particularly in deep cycle applications)
What is the difference between VRLA (Gel or AGM) batteries and traditional wet or flooded batteries?
- Wet or flooded batteries do not have special pressurized sealing vents, as they do not work on the recombination principle
- Wet batteries contain excess liquid electrolyte that can spill and cause corrosion if tipped or punctured
- Wet batteries should not be used near sensitive electronic equipment
- Wet batteries can only be installed “upright”.
What happens now to the oxygen as it makes its way to the negative plate is different in wet vented batteries than it is in VRLA batteries.
In wet or flooded lead-acid batteries of the vented design with excess electrolyte, it is practically impossible for the oxygen to move to the negative plate. Immediately after having left the positive plate, it bubbles up and escapes through the vent plug.
In VRLA batteries, a densely porous medium is offered to the oxygen to facilitate its movement. The porous medium in an AGM VRLA battery is the glass mat. The porous medium in a Gel VRLA battery is the cracks in the gelled electrolyte.
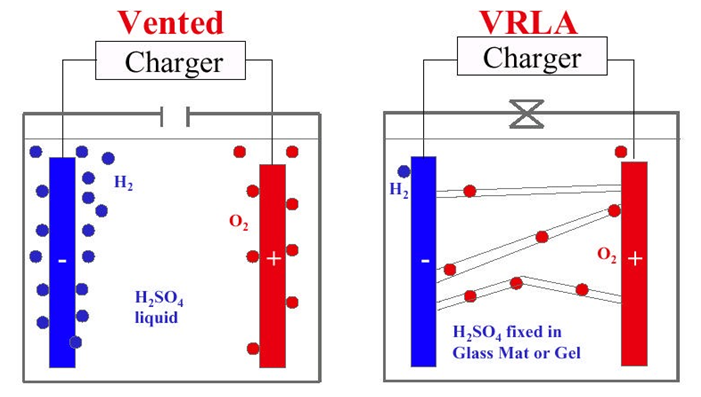
Figure 1 - Vented Battery Gassing and VRLA Battery Recombination
VRLA batteries are designed using proven gas recombination technology which removes the need for regular water addition by controlling the evolution of hydrogen and oxygen during charging. This means that the oxygen normally produced on the positive plates of all lead-acid batteries is absorbed by the negative plate through a porous medium (see Figure 1) without being vented. This suppresses the production of hydrogen at the negative plate. Water (H20) is produced instead, retaining the moisture within the battery. It never needs watering and should never be opened as this would “poison” the battery with additional oxygen from the air.
The retained oxygen produces an over-pressure within the cell. This is normal. The battery’s sealing valves should not open at too low pressure because this would allow too much oxygen to escape and be irretrievably lost. If the defined opening pressure is achieved, the sealing valve will open for a short time to release over-pressure caused by the accumulated gas. Under normal operating conditions this gas consists mainly of hydrogen. Under unfavorable conditions, (high charge voltages at high temperatures, for instance) oxygen would also escape.
Different types of batteries use test procedures that allow different end of life criteria. For example:
- An electric vehicle or standard deep cycle product would be considered to be at its end of life when it was not able to deliver 50% of its rated capacity
- For home UPS/ Inverter
- An electric vehicle or standard deep cycle product would be considered to be at its end of life when it was not able to deliver 60% of its rated capacity when discharged at 3 Hours Rate
How long a battery will last in terms of its usable life depends completely on how often the battery is discharged and charged, how fast or at what rate it is discharged and charge, to what extent it is discharged or the depth of discharge, and how well or properly it is recharged. Surrounding temperature of the batteries also impact the life of the battery. Higher temperature increases the performance of the battery but lowers the life cycle of the battery.
- If there is a 0.030 (30 “points”) or more difference in the specific gravity reading between the highest and lowest cells of a flooded battery. This means the flooded battery you are testing has a weak or dead cell(s)
- If battery is heating too much on uses
- If after full Charging battery Gravity is not maintained, and difference is more in each cell if electrolyte spillage & more water top-up is not happened
- If the battery will not recharge to a 75% or more state-of-charge level
- If a digital voltmeter indicates 0 volts on a battery, you know to have been charged properly, you have an open or short-circuited cell and you should replace the battery
- If a digital voltmeter indicates < 10.5 V volts on a battery you know to be a fully charged battery, then the battery probably has a shorted or dead cell
- If the battery is fully charged, check the float indicator showing green or a good voltmeter indication then you can test the capacity of the battery by applying a known load and measuring the time it takes to discharge the battery until 20% of its originally published capacity is remaining
Battery capacity |
maximum discharge capacity @ 80% discharge |
Charging option |
||
|---|---|---|---|---|
Only Grid charging |
Solar + Grid Charging |
Only Solar |
||
200Ah |
160 |
By default Inverter charging current set at 15A-16A, so in 10-11 Hrs battery will be fully charged. | 10 Hrs (If Grid power avilable on day time average 10 Hrs so minimum solar wattage=1.5* Battery Capacity) | Total Solar Panel Capacity= 3 time of Battery Capacity |
| 1) Battery charge by solar power and load run by grid. | Ex- 200Ah Battery charging need solar panel capacity= 3*200= 600 Watt. | |||
| 2) If solar is unsuffceint ,so Battery will charge by Solar+Grid charging | Ex- 100 W Solar Panel generate power = 100/17.5*80% = 4.5 AMP/ hour. Average Sun power is 6 Hrs , so 600 watt panel generate = 6*4.5*6 Total power is 162 Amp. | |||
| 3) Charging current is sharing basis, battery is charged 1st by Solar. If solar current is going down in morning & evening time then balance power will be taken by Grid. | ||||
Bulging is when battery sides take curvy shape(outside) due to non-passage of gases. Bulging (gas formation) happens due to the following reasons:
- Expansion of plates during charging and discharging process
- Over charging of battery
- Chocking of vent plugs thus blocking release of gas
- Impurities in alloy
Eastman TTMF & STMF battery do not bulge because:
We use of high-quality alloy resulting is very less gas formation.
We use specially design aqua trap vent plugs resulting is smooth release of gas.
Eastman T-Gel Batteries do not bulge because:
The electrolyte is “condense form of fumed silica gel” resulting in negligible gas formation.
Even for the negligible amount of gas to escape, air vents are placed which opens when a certain amount of gas pressure is produced in the battery.
The container is heavily reinforced (rib design) which gives it anti-bulge properties.
The charging pattern of Eastman Tubular Gel battery and SMF battery is same. But the upper charging cut off voltage is different.
For tubular battery the upper charging cut off voltage is 14.4V and for the SMF battery it is 14.0/14.2 Volt.
Due to this reason inverter manufacturer give option in inverters to charge the battery in SMF or Tubular mode.
Eastman batteries are to be charged in Tubular mode.
Measurement and troubleshoot
Maintenance
| Terminal Type | Torque (N.m) |
| L- Type | 8-10 N.m |
Rating
- A 150 AH battery at C20, will last for 20 hours on a load of 7.5 A.
- A 150 AH battery at C10 will last for 10 hours on a load of 15 A.
- A 150 AH battery at C5 will last for 5 hours at a load of 30 A.
Charging
Date Coding
xxxCH9xxxxxxxxxxx – 12/08/2019
4th character – Date (1,2,3…9, A, B, C…).
5th character - Month(A-Jan, B-Feb, L-Dec).
6thCharacter – Year (7- 2017, 8- 2018, …., 6- 2026).
Additives and external compositions
Battery Selection
- For long life inverter/ups application flooded tubular batteries are recommended.
- For high current discharge flat plate batteries are preferred.
What is the difference between a deep cycle battery, a starting battery, and a dual-purpose battery?
Common Mistakes
- Overcharging: Continuous charging causes accelerated corrosion of the positive plates, excessive water consumption, and in some cases, damaging temperatures within a lead acid battery.
- Under watering: In flooded batteries water is lost during the charging process. If the electrolyte level drops below the tops of the plates, irreparable damage may occur. Water levels should be checked and maintained routinely.
- Over-watering: Excessive watering of a battery results in additional dilution of the electrolyte, resulting in reduced battery performance. Additionally, watering the battery before charging may result in electrolyte overflow and unnecessary additional maintenance.
- DM water: It is recommended to use only demineralized or distilled water, Normal tap water should not use for battery, it will reduce the life of the battery.
Temperature Factor and Effects
Battery Recycling
- Every 1degree above 27º C add 7 points to the hydrometer reading.
Example: @ 32º C the hydrometer reads: 1.225 the actual reading: 1.225+ 0.0035 1.2285.
- For every ten degrees below 27º C subtract 7 points from the hydrometer reading.
Eastman recommends using the following:
- For every 1 C below 25º C add 0.005 volts per cell to the charger voltage setting.
- 2: A 12-volt battery @ 21º C. The recommended charging voltage at 25º C is 14.8 volts. The adjusted charging voltage is 14.8 + (6 cells * 4 degrees below * 0.005) = 14.92 volts.
- 2: A 12-volt battery @ 29.5º C. The recommended charger voltage at 25º C is 14.8 volts. The adjusted charging voltage is 14.8 – (6 cells * 4.5 degrees above * 0.005) = 14.67 volts.